Have you ever wondered about the seemingly complex world of organic chemistry? Behind the seemingly esoteric names and intricate structures lie fascinating compounds that form the building blocks of our everyday lives. Take 2-chloro-2-methylpropane, for instance. This compound, often nicknamed “tert-butyl chloride,” might sound like a chemical mystery, but it plays a surprisingly vital role in various industries and scientific endeavors. In this exploration, we’ll dive into the intriguing world of 2-chloro-2-methylpropane, uncovering its structure, properties, reactions, and the diverse applications that make it a significant player in modern-day chemistry.
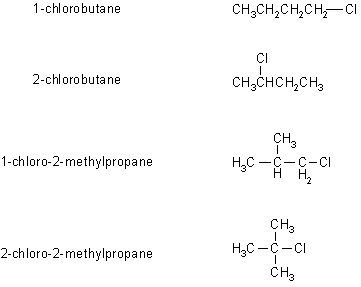
Image: www.chem.ucalgary.ca
2-Chloro-2-methylpropane, or tert-butyl chloride, is an organic compound with a seemingly simple structure, but one that holds a wealth of chemical intrigue. Its molecular formula, C4H9Cl, hints at its key components: carbon, hydrogen, and chlorine. The “tert-butyl” prefix indicates a specific arrangement of the carbon and hydrogen atoms, revealing its characteristic branched structure. This structure plays a crucial role in shaping its chemical behavior and reactions. Understanding the unique features of its structure provides a foundation for comprehending its various applications.
Delving into Structure and Properties
At the heart of 2-chloro-2-methylpropane lies a central carbon atom bound to three methyl groups (CH3) and one chlorine atom (Cl). This “quaternary carbon” arrangement, where a single carbon atom is bonded to four other carbons, is a hallmark of branched structures, giving the compound its distinctive shape and properties. The presence of the chlorine atom contributes to the molecule’s reactivity, influencing how it engages in chemical reactions.
A Molecular Dance: Shape and Reactivity
The shape of 2-chloro-2-methylpropane is not simply a matter of aesthetics. It impacts how the molecule interacts with other molecules, affecting its reactivity and how it behaves in reactions. The presence of the chlorine atom creates a region of electron density around the carbon atom to which it’s attached. This localized electron density makes the carbon atom more susceptible to attack by nucleophiles, which are electron-rich species that are attracted to positive charges. The branched structure also influences the ease with which the molecule can undergo various reactions, making it a valuable reagent in organic synthesis.
Physical Properties: A Closer Look
2-Chloro-2-methylpropane is a colorless, volatile liquid at room temperature, emitting a somewhat pungent odor. Its volatility is a consequence of its relatively weak intermolecular forces, arising from the absence of significant hydrogen bonding. It is only slightly soluble in water, a characteristic shared by many organic compounds, which tend to be more soluble in non-polar solvents. Understanding these basic physical properties helps us discern how it reacts and interacts with other substances.
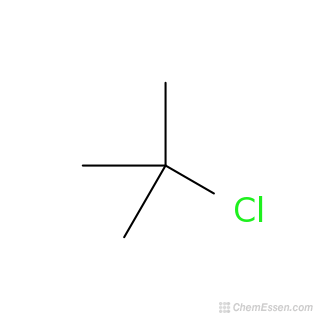
Image: www.molinstincts.com
Unveiling the Chemical Reactions: A World of Transformations
The most common reactions involving 2-chloro-2-methylpropane primarily revolve around its ability to undergo substitution and elimination reactions, taking advantage of its unique structural features. These reactions are fundamental to organic chemistry and play a critical role in the formation of new and useful compounds.
Substitution Reactions: A Tale of Replacement
In substitution reactions, the chlorine atom in 2-chloro-2-methylpropane is replaced by another atom or group. This happens through a process called nucleophilic substitution, where a nucleophile, an electron-rich species, attacks the carbon atom bearing the chlorine atom. The chlorine atom, a good leaving group, departs as a chloride ion, giving rise to a new compound. For example, 2-chloro-2-methylpropane can react with hydroxide ions (OH-) to produce tert-butyl alcohol (C4H9OH) in a nucleophilic substitution reaction.
Elimination Reactions: A Journey of Removal
Another key type of reaction involving 2-chloro-2-methylpropane is elimination. In elimination reactions, a molecule loses atoms or groups, leading to the formation of a double bond. In the case of 2-chloro-2-methylpropane, one of the hydrogen atoms on a neighboring carbon atom and the chlorine atom are removed, leading to the formation of isobutene (C4H8). This process is particularly relevant in the synthesis of various organic molecules containing double bonds.
Applications: From Industry to Academic Laboratories
The versatility of 2-chloro-2-methylpropane extends beyond the realm of theoretical chemistry. It finds applications across various industries, showcasing its importance in the synthesis of essential products and its role in scientific research.
Industrial Applications: A Bridge Between Chemistry and Everyday Life
2-Chloro-2-methylpropane is a critical ingredient in the production of polymers. It serves as a key monomer in the synthesis of isobutylene polymers, which are used to create a range of products, from adhesives and sealants to synthetic rubbers and even medical devices. Its presence in these products highlights its connection to our daily lives, touching multiple aspects of modern society.
The compound also plays a part in the preparation of various pharmaceuticals, where its unique reactivity enables the synthesis of essential medications for treating various ailments. Its ability to readily undergo reactions with other compounds makes it a valuable tool in the pharmaceutical industry, contributing to the development of new drugs.
Research Applications: Unveiling the Unknown
Beyond industrial applications, 2-chloro-2-methylpropane is a crucial reagent in academic research. Scientists utilize it in laboratories worldwide to explore diverse chemical reactions and investigate the mechanisms underlying these transformations. Its reactivity and readily available nature make it a favorite among organic chemists. From probing the nuances of reaction mechanisms to exploring the synthesis of novel compounds, 2-chloro-2-methylpropane continues to fuel scientific discovery in research labs.
Safety Considerations: Navigating with Prudence
While 2-chloro-2-methylpropane serves as a valuable tool in numerous fields, it is crucial to handle it with caution. It is a flammable and volatile liquid, requiring proper storage and handling techniques. Exposure to high concentrations can cause respiratory irritation and other health concerns, emphasizing the importance of appropriate precautions. Always consult safety data sheets and follow lab protocols to ensure a safe and healthy work environment when using this compound.
Future Trends: A Glimpse into the Horizon
The study of 2-chloro-2-methylpropane is continuously evolving, fueled by advancements in computational chemistry and improved analytical techniques. Researchers are exploring new and sustainable methods for its synthesis, aiming to minimize environmental impact and optimize efficiency. The development of new catalysts and reaction conditions for its utilization in industrial processes is also a growing area of interest, potentially leading to new applications and improved product yields. The future of 2-chloro-2-methylpropane research holds the promise of further innovation, potentially unlocking new applications and enhancing its impact on numerous industries.
2 Chloro 2 Methylpropane
Final Thoughts: A Journey Through the Realm of Chemistry
Our journey into the world of 2-chloro-2-methylpropane has revealed its intriguing structure, its versatility in chemical reactions, and its significant roles in various sectors. From industrial production lines to academic laboratories, this compound has solidified its place in modern chemistry. Understanding its basic properties and reactions provides a springboard for appreciating its diverse applications and the potential for further discoveries in the years to come. So, the next time you encounter this seemingly innocuous chemical name, remember that it represents a world of intriguing chemical transformations and applications that are shaping our world.
If you’re curious to learn more about 2-chloro-2-methylpropane or other fascinating compounds in organic chemistry, don’t hesitate to explore reputable scientific resources, engage in online forums, and delve into the vast body of research available. The journey of chemical exploration is filled with wonder, challenging our perspectives and revealing the intricate connections between the molecular world and our everyday lives.





