Remember that time you were studying chemistry in high school and had no idea what the teacher was talking about when they started discussing molecular orbitals? It felt like a whole new language was being thrown at you, right? Well, let’s face it; understanding molecular orbitals can be tricky. That’s why we’re here today to dive into the world of C2-MO diagrams! Don’t worry, we’ll break it down in a way that makes sense and will leave you feeling confident about molecular bonding.
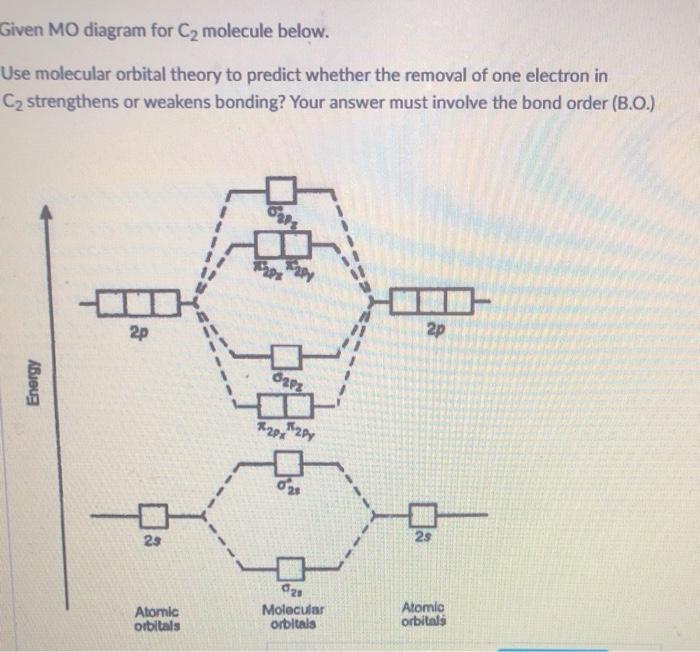
Image: www.chegg.com
This article is your ultimate guide to understanding C2-MO diagrams. As we explore the concept of molecular orbitals and their representations through diagrams, you’ll discover how these tools help us understand the intricate nature of chemical bonds and the behavior of molecules. Get ready to demystify the world of molecular orbitals.
What are C2-MO Diagrams and Why are They Important?
Before we delve into the specifics of C2-MO diagrams, let’s start by defining molecular orbitals themselves. They represent regions in a molecule where electrons are likely to be found. Unlike atomic orbitals, which are associated with individual atoms, molecular orbitals encompass the entire molecule. Remember those beautiful balloon-like orbitals you learned about in introductory chemistry? That’s what we’re talking about here, but for the entire molecule this time.
C2-MO diagrams are visual representations of these molecular orbitals in a molecule with two identical atoms, such as diatomic molecules like O2 or N2. They show how atomic orbitals combine to form molecular orbitals and how these orbitals are filled with electrons, which determines how strongly the atoms in the molecule are held together.
Delving into the Construction of C2-MO Diagrams
Let’s break down the construction of C2-MO diagrams. It all starts with the atomic orbitals of the individual atoms. We use a process called Linear Combinations of Atomic Orbitals (LCAO) to understand how these atomic orbitals interact to form molecular orbitals. For a C2-MO diagram, we consider the valence orbitals of the two carbon atoms.
The C2-MO diagram is essentially a visual map of how these atomic orbitals combine. The most common orbital combinations involve the interaction of the 2s and 2p orbitals. Remember, we’re dealing with two carbon atoms, so we’ll have two sets of atomic orbitals that interact. These orbital interactions result in two main types of molecular orbitals: sigma (σ) and pi (π) orbitals.
Sigma (σ) Orbitals, which are formed by the head-on overlap of atomic orbitals, are symmetrical about the internuclear axis, which is the imaginary line connecting the two atomic nuclei. Imagine two little balls (atomic orbitals) coming together and overlapping directly. They form a long, sausage-shaped orbital that has rotational symmetry.
Pi (π) Orbitals are formed by the side-by-side overlap of atomic orbitals and are perpendicular to the internuclear axis. Picture two atoms side-by-side, with their atomic orbitals like two saucers facing each other. The overlapping region gives us a more donut-like shape. Pi orbitals have this nice symmetry where you can rotate them around the internuclear axis without breaking the symmetry.
Understanding the Different Types of Molecular Orbitals
C2-MO diagrams are crucial for understanding the characteristics of molecules. The different types of molecular orbitals formed have distinct energy levels and bonding properties, which affect a molecule’s overall stability, polarity, and reactivity.
1. Bonding Orbitals: These orbitals are lower in energy than the original atomic orbitals and contribute to bonding. Think of them as the glue holding the atoms together. They are usually filled with electrons to create a stable bond between the atoms.
2. Antibonding Orbitals: These orbitals are higher in energy than the original atomic orbitals and weaken the bond between atoms. They are usually empty or have electrons in them only for excited states. Imagine them like a force that pushes the atoms apart.
3. Nonbonding Orbitals: These orbitals have the same energy as the atomic orbitals from which they originate. They are neither directly involved in bonding nor antibonding and often play a secondary role in molecular behavior.

Image: schematicginglymi.z14.web.core.windows.net
How to Draw a C2-MO Diagram
Drawing C2-MO diagrams can be a little daunting. However, it becomes easier when we understand a few key steps:
- Start with the atomic orbitals: Identify the valence orbitals of the two atoms involved.
- Combine the orbitals: Use the LCAO method to determine the molecular orbitals that result from the interaction of the atomic orbitals, remembering to account for the symmetry properties of the orbitals.
- Energy levels: Arrange the molecular orbitals in order of increasing energy. Bonding orbitals will be lower in energy than antibonding orbitals.
- Fill the orbitals: Fill the molecular orbitals with electrons, following Hund’s rule and the Pauli exclusion principle. Remember that you need to account for all the valence electrons from both atoms.
- Label the orbitals: Label each molecular orbital with its appropriate symbol (σ, π, σ*, π*) and indicate whether it is bonding, antibonding, or nonbonding.
Interpreting a C2-MO Diagram
Once you’ve constructed a C2-MO diagram, you can interpret its information to predict the properties of the molecule:
1. Bond Order: The bond order is the number of electron pairs shared between two atoms. You can calculate it by subtracting the number of electrons in antibonding orbitals from the number of electrons in bonding orbitals and then dividing by two. A higher bond order generally indicates a stronger bond.
2. Magnetic Properties: A C2-MO diagram helps determine if a molecule is diamagnetic (no unpaired electrons) or paramagnetic (has unpaired electrons).
3. Reactivity: The distribution of electrons in the molecular orbitals affects the molecule’s reactivity and stability.
Tips and Expert Advice for C2-MO Diagram Mastery
Understanding C2-MO diagrams can unlock a whole new world of insights into chemical bonding. Here are a few tips to help you master this concept:
1. Practice: The best way to learn is by doing. Draw diagrams for different diatomic molecules and practice interpreting their features. Start with simple examples and gradually move towards more complex molecules. Remember, there are many resources available online and in textbooks that can provide you with practice examples.
2. Visualization: Try to visualize the overlap of atomic orbitals and how they form the molecular orbitals. Different types of software can help you visualize these interactions and create 3D representations. These visuals greatly increase understanding because they offer a very clear and engaging approach to learning.
3. Connect with Others: Don’t hesitate to ask for help from your instructors, classmates, or online communities. Sharing knowledge and discussing these concepts can be a big benefit. Remember, even experts started somewhere, so seeking help is part of the learning process and is definitely encouraged.
FAQs About C2-MO Diagrams
Q: What is the difference between bonding and antibonding orbitals?
A: Bonding orbitals are lower in energy than the original atomic orbitals and contribute to bonding. Antibonding orbitals are higher in energy and weaken the bond.
Q: How do I know which atomic orbitals will combine to form molecular orbitals?
A: You can usually focus on the valence orbitals of the atoms, which are the ones that are most involved in bonding. The overlap of the atomic orbitals determines the shape and symmetry of the resulting molecular orbitals.
Q: What are some examples of diatomic molecules that can be represented by C2-MO diagrams?
A: Common examples include H2, N2, O2, F2, Cl2, Br2, and I2.
C2- Mo Diagram
Conclusion
C2-MO diagrams provide a powerful tool for understanding the nature of chemical bonding. By considering how atomic orbitals interact and how these interactions are portrayed visually, we gain invaluable insight into the fascinating world of molecular structure and behavior. We hope this guide has demystified the concept of C2-MO diagrams and empowered you to explore their applications further.
Are you interested in further exploring the world of molecular orbitals, their applications in chemistry, and their implications for different fields? Let us know in the comments below





