Have you ever wondered about the secrets hidden within the structure of a seemingly simple molecule like BrF3? It might appear insignificant at first glance, but delve deeper, and you’ll discover a fascinating world of molecular geometry, electron distribution, and the intriguing concept of polarity. Today, we’re embarking on a journey to unravel the mysteries of BrF3, exploring whether it’s a polar molecule or prefers the company of its nonpolar counterparts.
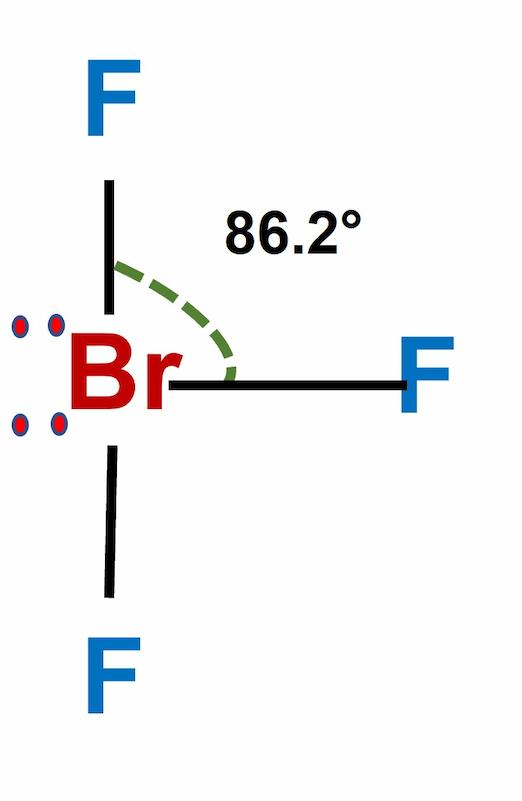
Image: whatsinsight.org
The significance of understanding the polarity of molecules extends far beyond the realm of textbooks and scientific journals. It plays a pivotal role in determining the properties of substances, influencing their behavior in various chemical reactions, and even shaping the world around us. From the way water dissolves substances to the properties of medications, understanding polarity is crucial for making sense of the intricate dance of molecules that governs our world.
Delving into the World of Molecular Geometry and Polarity
Before we tackle the question of BrF3’s polar nature, let’s equip ourselves with the essential tools to understand this intriguing concept. The shape of a molecule, its molecular geometry, holds the key to unraveling its polarity. Imagine a molecule as a group of atoms connected by bonds, like a miniature architectural structure. Each atom possesses a nucleus surrounded by a cloud of negatively charged electrons. The distribution of these electrons within the molecule is what determines its polarity.
Think of it this way: If the electrons are evenly distributed, the molecule is like a perfectly balanced seesaw, with no bias towards one side. This is characteristic of a nonpolar molecule. However, if the electron cloud leans towards one side, the molecule becomes unbalanced, creating a dipole moment – a separation of charge. This lopsided electron distribution gives rise to a polar molecule, one that possesses a positive and a negative pole like a tiny magnet.
The Case of BrF3: A Tale of Lone Pairs and Bent Shapes
Now, let’s turn our attention to our molecular protagonist, BrF3. This molecule comprises one bromine atom (Br) and three fluorine atoms (F). To decipher its polarity, we must first understand its geometry.
The central bromine atom, with its seven valence electrons, forms three single bonds with fluorine atoms. The remaining two valence electrons exist as a lone pair on the bromine atom. This lone pair, along with the three Br-F bonds, dictate the shape of the molecule.
Remember, electrons, being negatively charged, repel each other. To minimize repulsion, the three fluorine atoms and the lone pair adopt a specific spatial arrangement, leading to a “T-shaped” molecular geometry. This T-shaped geometry signifies that the three fluorine atoms are not distributed symmetrically around the bromine atom. The lone pair, with its greater electron density, exerts a stronger repulsive force, pushing the fluorine atoms slightly away from each other.
The Verdict: BrF3 is Polar
The T-shaped structure of BrF3 directly influences its polarity. The asymmetrical arrangement of the fluorine atoms and the lone pair creates an uneven distribution of electrons. The bromine atom, with the lone pair, carries a slight negative charge, while the fluorine atoms, pulling a bit of the electron density towards themselves, become slightly positive. This uneven charge distribution results in a net dipole moment, confirming that BrF3 is indeed a polar molecule.
Think of it like a tug-of-war between the bromine atom and the fluorine atoms. The bromine atom, with its lone pair, holds a slight advantage, pulling the electron cloud closer to itself, resulting in a polarity similar to a magnet with two distinct ends – a positive pole and a negative pole.
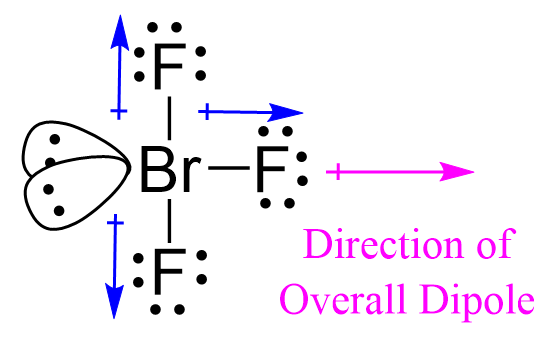
Image: general.chemistrysteps.com
The Impact of Polarity: Understanding BrF3’s Behavior
The fact that BrF3 is a polar molecule has significant implications for its behavior and interactions. The dipole-dipole forces that exist between polar molecules influence their properties, such as melting point, boiling point, and solubility.
Polar molecules like BrF3 tend to dissolve well in other polar solvents, such as water. The positive and negative ends of the molecules attract each other, leading to a favorable interaction. In contrast, BrF3 would be less soluble in nonpolar solvents like oil, where the lack of charge separation would hinder their interaction.
Furthermore, the dipole moment in BrF3 can influence its reactivity. The electron density shift towards the bromine atom could make it more susceptible to attack by electrophiles, molecules that are attracted to regions of high electron density.
Beyond BrF3: The Importance of Understanding Molecular Geometry and Polarity
Our exploration of BrF3 highlights the profound connection between a molecule’s structure and its properties. The seemingly simple concept of polarity carries far-reaching consequences, influencing the behavior of substances, shaping the interactions between molecules, and playing a crucial role in countless chemical processes.
From the pharmaceutical industry, where the polarity of drugs influences their absorption and efficacy, to the design of new materials with specific properties, understanding polarity is an indispensable tool for scientists and engineers alike.
Brf3 Polar Or Nonpolar
Next Steps: Unveiling the World of Molecular Polarity
This journey into the world of BrF3 has only scratched the surface of the fascinating topic of molecular polarity. Further exploration will reveal how the interplay of molecular geometry, electron distribution, and dipole moments governs the behavior of countless molecules.
We encourage you to delve deeper into the world of chemistry. Explore resources like textbooks, scientific journals, and online platforms to gain a deeper understanding of this fundamental concept. You can also conduct simple experiments at home to visualize the effects of polarity, such as observing the interaction between water and oil or exploring the solubility of different solutes. Remember, the world of molecules is a rich tapestry of hidden secrets waiting to be unraveled.
So, keep asking questions, keep exploring, and keep learning about the fascinating world of chemistry.





