Imagine a world without propane. No cozy evenings by the fireplace, no backyard barbecues, no warm showers on chilly mornings. Propane, a ubiquitous fuel, plays a vital role in our lives, powering everything from homes and businesses to vehicles and even some industrial processes. But at the heart of this versatile fuel lies a fascinating concept: its molar mass.
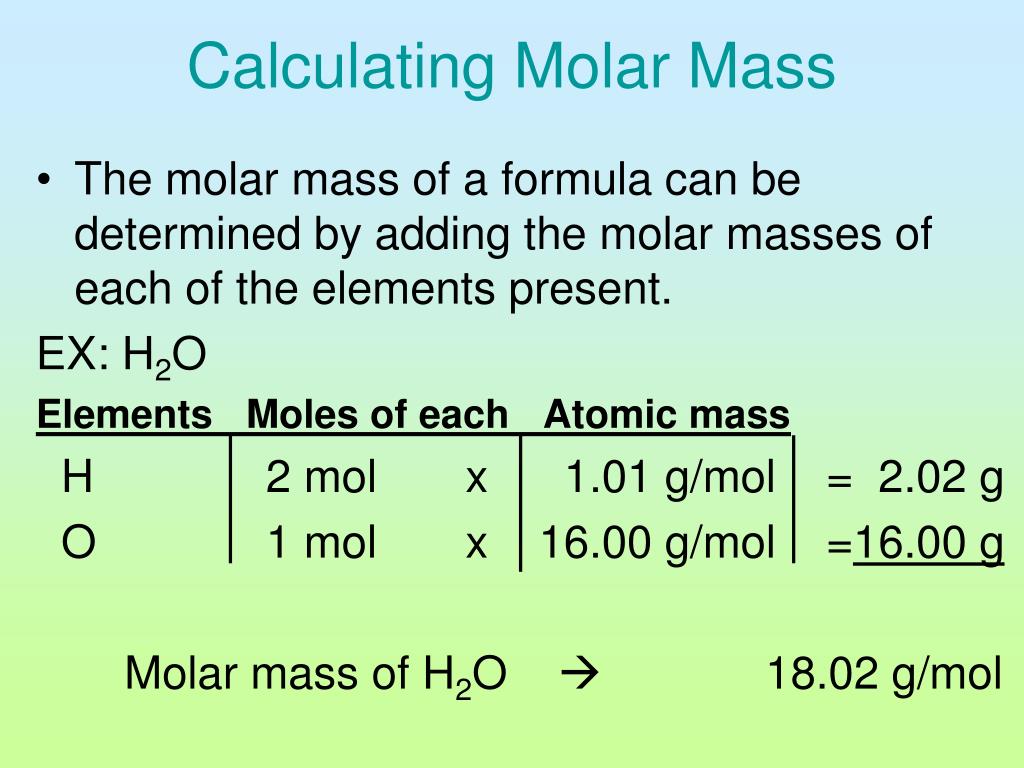
Image: haipernews.com
Understanding the molar mass of propane is crucial for comprehending its properties, reactions, and applications. This seemingly simple concept unlocks deeper insights into the world of chemistry and ultimately empowers you to make informed decisions related to propane usage. This journey into the heart of propane’s molar mass will not only enhance your knowledge but also equip you with a greater appreciation for the science behind this essential fuel.
Delving into the Molar Mass of Propane
The molar mass of a substance is the mass of one mole of that substance. Imagine a mole as a specific quantity – like a dozen for eggs, but for molecules. Just like a dozen always contains 12 eggs, a mole always contains 6.022 x 10^23 molecules – a massive number called Avogadro’s number.
To determine the molar mass of propane (C3H8), we need to consider the individual atomic masses of its constituent elements, carbon and hydrogen. The periodic table, the chemist’s bible, provides these atomic masses: carbon (C) has an atomic mass of approximately 12.011 atomic mass units (amu), and hydrogen (H) has an atomic mass of approximately 1.008 amu.
Step-by-step, here’s how to calculate the molar mass of propane:
-
Identify the chemical formula: Propane’s chemical formula is C3H8, indicating it has three carbon atoms and eight hydrogen atoms.
-
Multiply the number of atoms of each element by its atomic mass:
- Carbon: 3 atoms * 12.011 amu/atom = 36.033 amu
- Hydrogen: 8 atoms * 1.008 amu/atom = 8.064 amu
-
Add the results to find the total molar mass:
- 36.033 amu + 8.064 amu = 44.097 amu
Therefore, the molar mass of propane is approximately 44.097 grams per mole (g/mol).
The Significance of Molar Mass in Propane Applications
The molar mass of propane is not just an academic concept. It plays a crucial role in various aspects of propane usage, including:
-
Storage and Transportation: Knowing the molar mass helps engineers design safe and efficient storage tanks and transport pipelines. The density and volume of propane can be calculated based on its molar mass, ensuring secure handling.
-
Combustion Reactions: The molar mass of propane is crucial for balancing chemical equations, particularly those related to propane combustion. This knowledge is vital for optimizing fuel efficiency, minimizing emissions, and ensuring complete combustion.
-
Energy Calculations: The molar mass of propane is instrumental in determining the heat energy generated during its combustion. This information is crucial for industries using propane for heating, power generation, and other processes.
Molar Mass: A Cornerstone of Chemistry
The concept of molar mass transcends propane and extends to all substances. It is the bridge connecting the atomic world with the macroscopic world we experience. Understanding molar mass empowers us to comprehend chemical reactions, calculate quantities used in chemical processes, and analyze the properties of various substances.

Image: www.slideserve.com
Beyond Propane: The Molar Mass in Everyday Life
The implications of molar mass extend far beyond propane. Think about the air we breathe. The molar mass of oxygen (O2) and nitrogen (N2) governs their concentrations in the atmosphere, influencing our breathing and survival.
Even in food, the molar mass of individual components plays a role in flavor and texture. The molar mass of sugar, for instance, affects its sweetness and how it interacts with other food ingredients.
Molar Mass Of Propane
Conclusion: A World Built on Atoms and Molar Mass
The molar mass of propane, though seemingly a simple concept, is a cornerstone of chemistry. It provides a fundamental understanding of how matter behaves and empowers us to utilize this knowledge across diverse fields. Whether you’re a scientist exploring the intricacies of chemical reactions, an engineer designing efficient energy systems, or simply a homeowner enjoying a propane-powered barbecue, the concept of molar mass shapes our world in countless ways.
So, the next time you encounter propane, remember the unseen forces at play – the atoms, their interactions, and the molar mass that governs their behavior. This understanding will enrich your appreciation for the science behind our everyday experiences.





