Have you ever wondered why a blacksmith plunges a glowing piece of metal into a bucket of water, or how a seasoned chef quenches a hot skillet in sizzling oil? It’s all about quenching – a heat treatment process that rapidly cools a heated material, transforming its properties. But the choice of quench medium – water vs. oil – can drastically alter the outcome, impacting the metal’s hardness, strength, and even its internal structure.
This article delves into the fascinating world of quenching, unraveling the science behind water and oil quenches, exploring their unique characteristics, and understanding how these seemingly simple fluids can revolutionize metal properties. Whether you’re a curious student, a budding engineer, or simply fascinated by the marvels of materials science, join us on this journey into the heart of heat treatment.
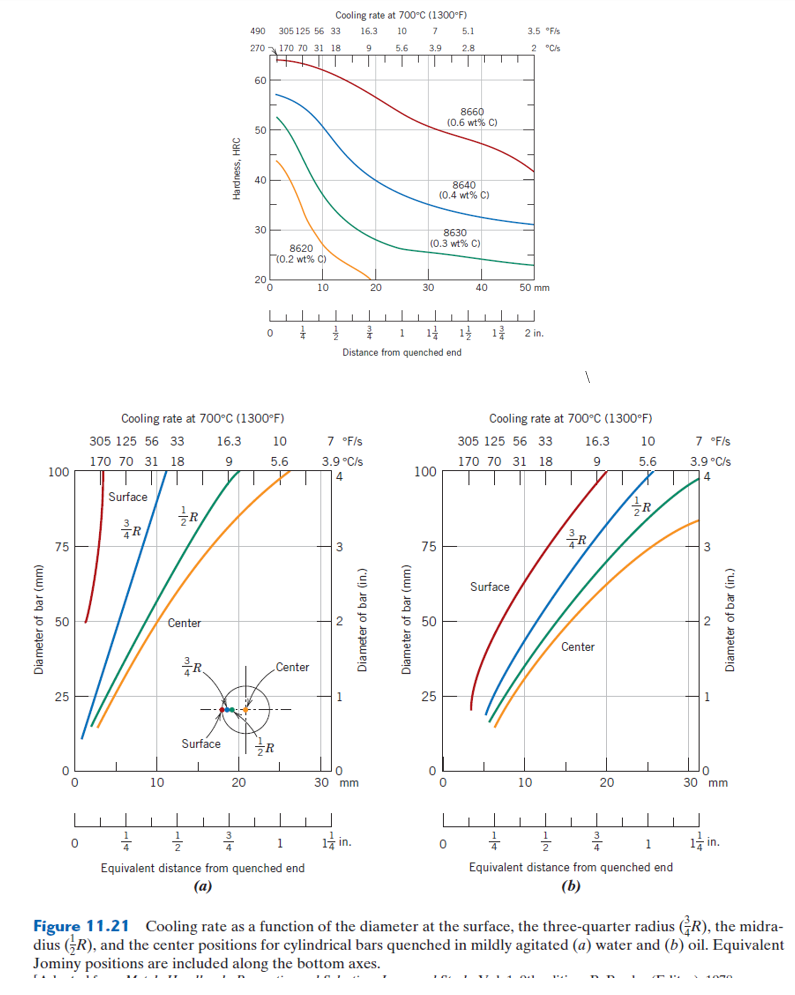
Image: www.chegg.com
The Essence of Quenching
Quenching is a fundamental step in heat treatment processes, particularly for steels. It’s the rapid cooling of a metal after it has been heated to a specific temperature. This controlled cooling alters the metal’s microstructure, dramatically influencing its final properties. Imagine a metal as a bustling city, its atoms arranged in a haphazard, disorganized way when heated. Quenching acts like a sudden, forceful storm, causing the atoms to scramble and freeze in their new, more organized positions, much like a city’s chaotic residents adapting to a sudden lockdown.
Water Quench: The Forceful Cooler
Water is a classic quenching medium, readily available and effective at rapidly extracting heat from hot metals. Its high thermal conductivity – its ability to transfer heat – allows it to cool the metal quickly. The rapid cooling rate associated with water quenching can make the steel significantly harder and stronger, much like a well-trained athlete pushing their limits.
Why Water?
- Rapid Cooling: The high heat transfer rate of water leads to a swift reduction in temperature, promoting the formation of harder, stronger microstructures.
- Cost-Effectiveness: Water is abundant and inexpensive, representing a practical quenching solution across various applications.
- Versatility: Water’s effectiveness is versatile, suitable for quenching diverse metals and metal alloys.

Image: oilwoyabushi.blogspot.com
But, There’s a Catch!
- Thermal Shock: The intense temperature drop can lead to thermal shock, potentially causing warping, cracking, or even shattering of thinner or brittle parts.
- Hydrogen Embrittlement: Water can contribute to hydrogen embrittlement, a phenomenon where hydrogen atoms can infiltrate the metal structure, making it more prone to cracking under stress.
Oil Quench: The Controlled Cooling Master
Oil quenches offer a more controlled cooling process, mitigating the harsh effects of water. Oils have significantly lower thermal conductivity than water, resulting in a slower cooling rate. This controlled cooling process allows for greater control over the final properties of the metal, much like a skilled sculptor carefully shaping a piece of clay.
Why Oil?
- Controlled Cooling: The slower cooling rate allows for a more gradual transformation of the metal’s microstructure, reducing the risk of thermal shock.
- Wider Range of Applications: Oils offer a broader range of quenching options, suitable for larger, more complex metal structures.
- Reduced Hydrogen Embrittlement: By significantly reducing the amount of hydrogen absorbed by the metal, oils minimize the risk of hydrogen embrittlement.
But, Be Aware:
- Slower Cooling: While oil offers greater control, the slower cooling rate can result in a softer, less hard metal. The final properties achieved depend heavily on the specific oil type and quenching temperature.
- Flammability: Oils are flammable substances, requiring careful handling and proper safety precautions in the workplace.
Water vs. Oil: Choosing the Perfect Quench
The choice between water and oil quenching is ultimately dictated by the desired properties of the final product.
Water Quenches are ideal for:
* Achieving maximum hardness for tools, cutting edges, and other high-strength applications.
* Processing smaller, less complex metal components.
Oil quenches are preferred for:
* Achieving a balance of hardness and toughness for larger, complex parts.
* Minimizing the risk of cracking and warping during quenching.
Quenching Beyond Water and Oil
While water and oil dominate the quenching landscape, new innovative quench media are emerging to further enhance and refine the process.
Polymer Quenches: The New Wave
- Polymer quenches, like those with polyethylene glycol (PEG), offer controlled cooling rates, comparable to oil-based quenches.
- They excel in minimizing distortion and cracking, making them ideal for complex shapes.
Salt Baths: For More Extreme Applications
- Salt bath quenching is particularly valuable for applications that require uniform temperature distribution across the metal.
- It’s commonly used in heat treatment processes requiring precise control and extremely high cooling rates.
As technology evolves, we can expect even more innovative quenching techniques to emerge, pushing the boundaries of metal properties and creating a new generation of stronger, more durable materials.
Water Vs Oil Quench
Conclusion
Quenching, a pivotal process in the world of heat treatment, holds the key to unlocking the full potential of metals. From the forceful cooling of water quenches to the controlled approach of oil quenches, the choice of quench medium determines the final properties of the metal, impacting its hardness, strength, and even its internal structure. Understanding the intricate interplay between quenching methods and metal behavior empowers engineers and material scientists to create components that meet the demands of diverse industries. So, the next time you encounter a metal object, take a moment to appreciate the subtle yet powerful role that quenching plays in its creation – a testament to the ingenuity and innovation that shape our world.





